Celecor Therapeutics
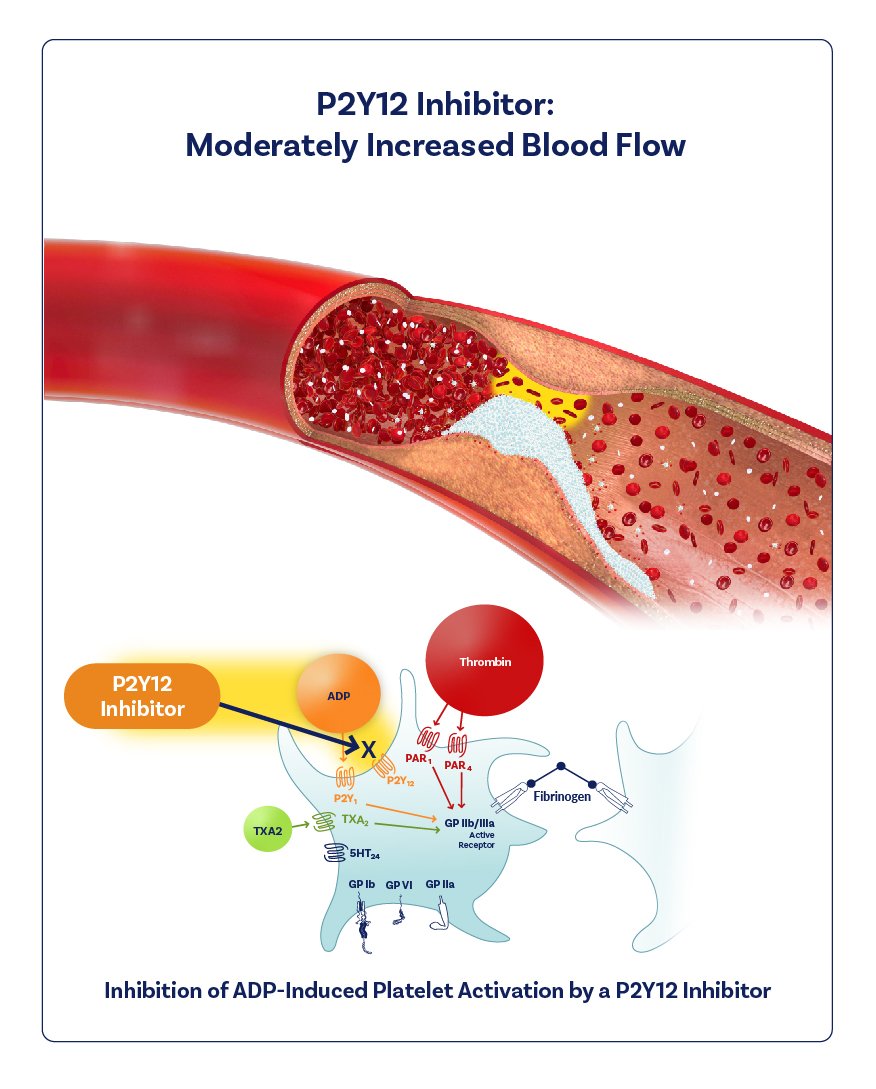
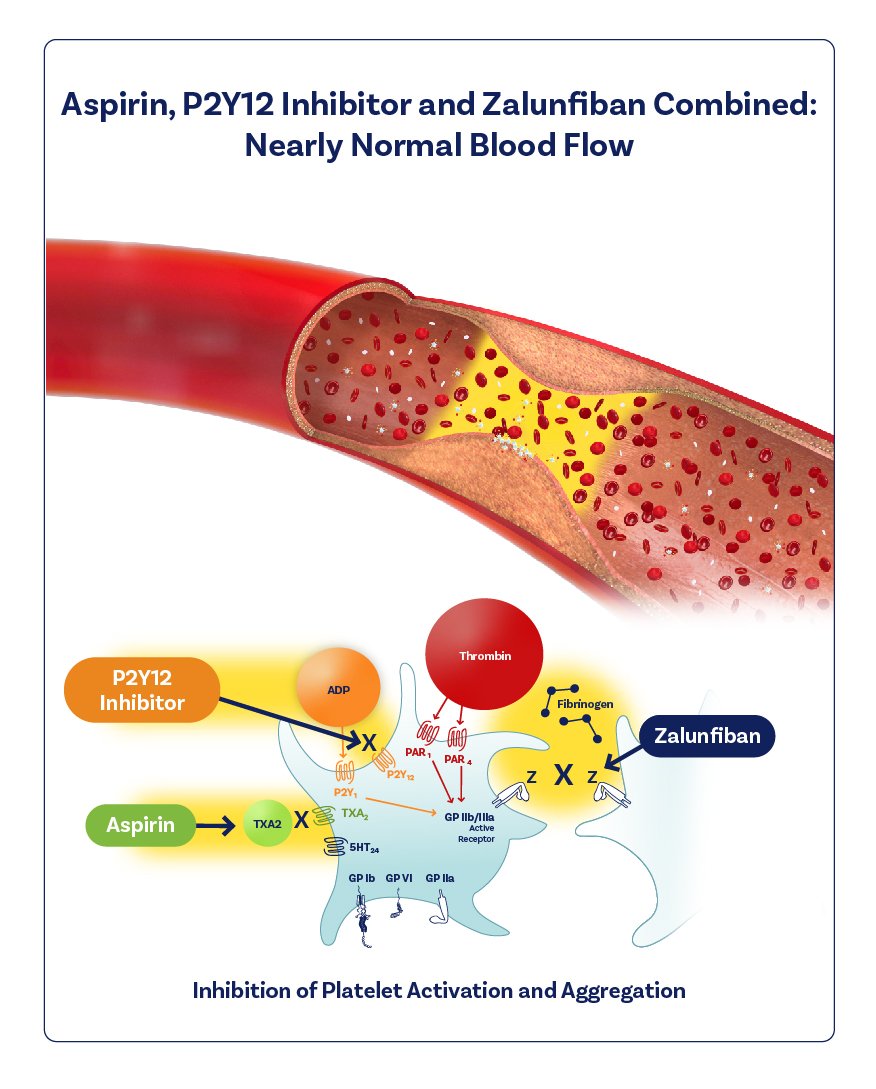
I illustrated the mechanism of action of a new drug in its final clinical trial phase called Zalunfiban.
Zalunfiban is a next-generation investigational GPIIb/IIIa platelet inhibitor for rapid pre-hospital treatment of STEMI heart attacks. I also created the branded graphics.
I worked closely with physicians, science writers, marketers, and researchers to accurately depict the mechanism of action. I collaborated with them to understand the specific visualization needs and to ensure that the illustration was scientifically accurate. Overall, I played a crucial role in visually communicating complex medical and scientific information, helping to enhance understanding and communication of the drug’s mechanism of action. I also created branded graphics for the backgrounder document and for the website.


50% of heart-attack deaths occur before a patient reaches the hospital.
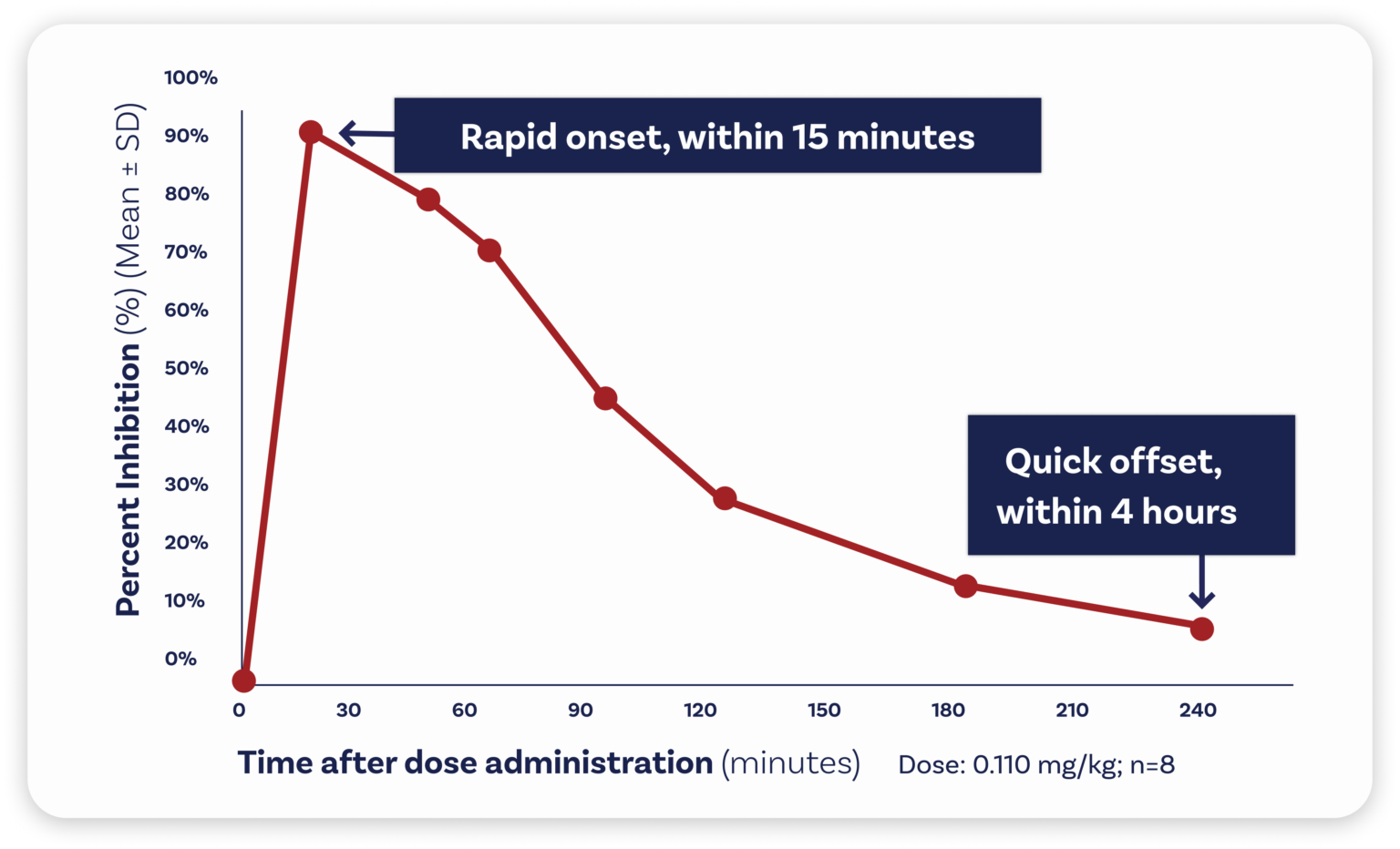
Zalunfiban is a next-generation investigational GPIIb/IIIa platelet inhibitor that was specifically designed for pre-hospital treatment of heart attacks and is injected subcutaneously (under the skin). It provides rapid, high-grade, limited-duration platelet inhibition within minutes. Zalunfiban reaches maximal effect within 15 minutes and has a half-life of about two hours, which may reduce the risk of bleeding and avoid interfering with later patient treatment.
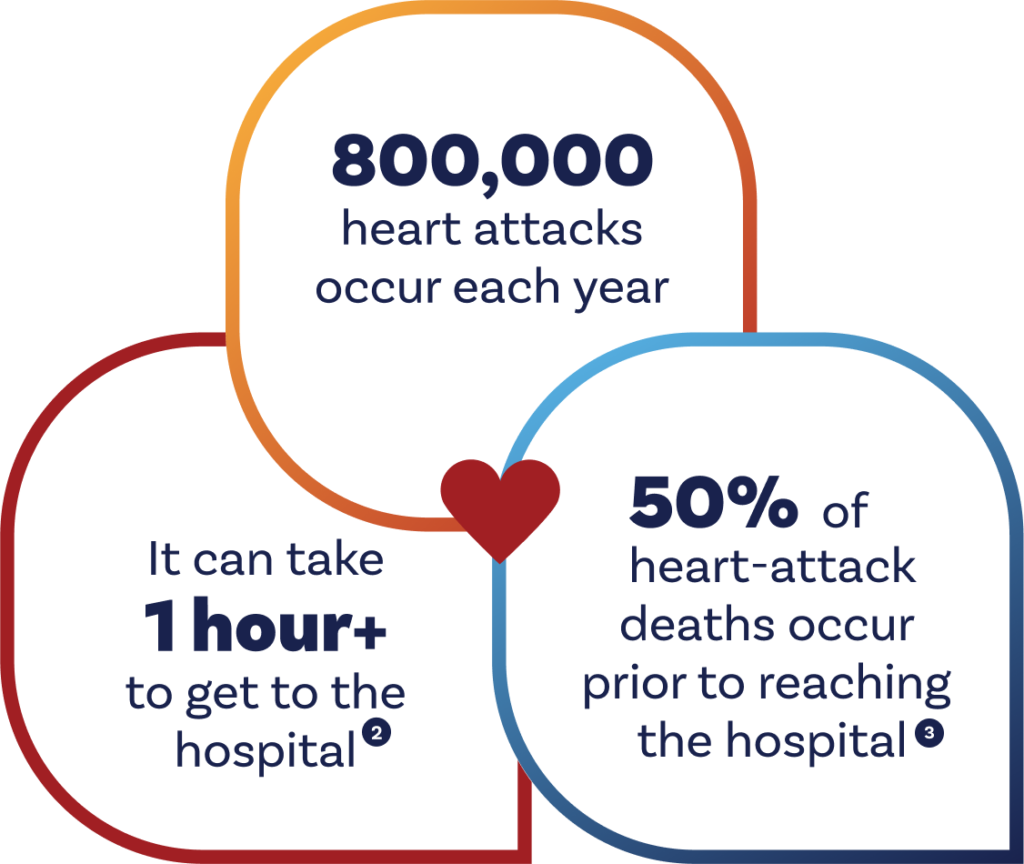
While in-hospital management of heart attacks has greatly improved over the years, treatment at the scene of a heart attack and during transport has stagnated. The extent of irreversible heart-muscle damage increases with every minute the blood vessel remains closed. This damage can later result in heart failure, one of the most common causes of hospitalization and death in the U.S. Zalunfiban is designed to treat STEMI heart attacks—the most severe form of heart attack, where blood flow to a portion of the heart is almost always cut off by a blood clot. About 40% of heart attacks are STEMIs.

